DNA-based “liquid biopsy” could help save lives and reduce health disparities
BY NIH
Researchers have developed a blood test that could make it possible for doctors to detect—then quickly prevent or slow down—acute heart transplant rejection, a potentially deadly condition that occurs in the early months after a patient has received a donor heart. They estimate that the test could eliminate up to 80% of invasive heart tissue biopsies currently used to detect rejection.
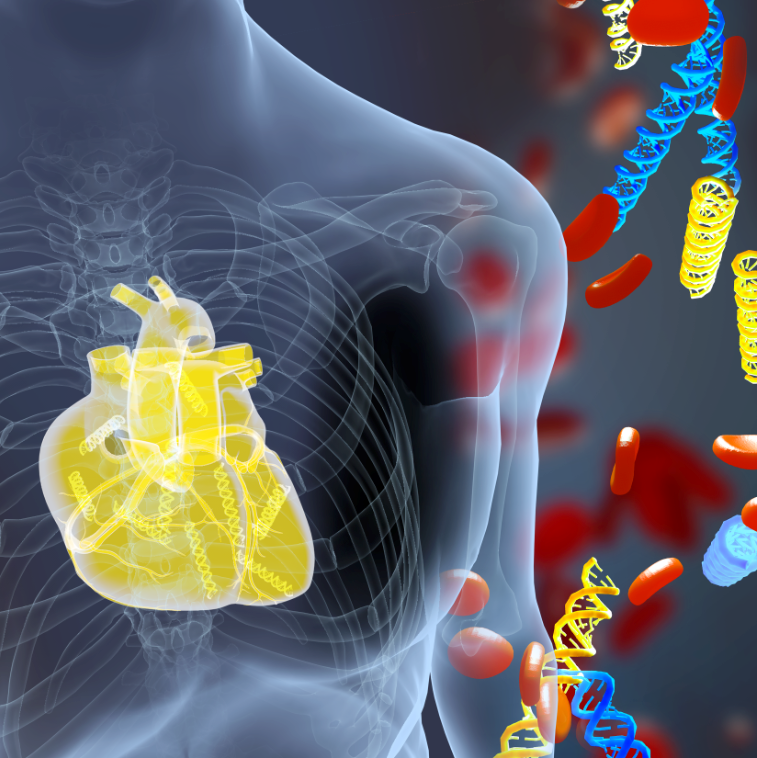 |
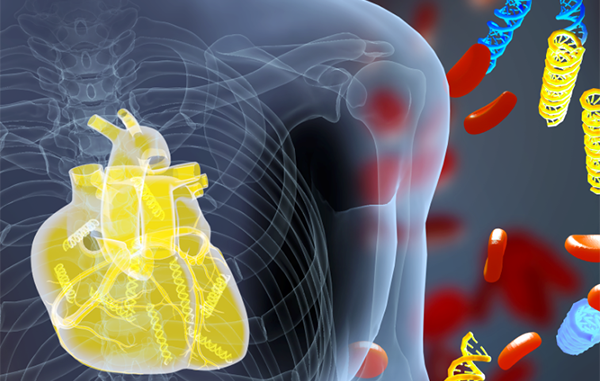
| Illustration shows DNA fragments (yellow) derived from a transplanted heart alongside the patient’s own DNA (blue). A new blood test measures donor DNA fragments and detects acute heart transplant rejection earlier than current methods. Credit: Erina He, NIH Medical Arts |
In studies of a group of nearly 200 heart transplant recipients, the new blood test performed better than tissue biopsies, as it signaled problems even when no outward signs of rejection were evident.
The study was primarily funded by the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health. The study’s findings are scheduled to appear January 13 in Circulation, a publication of the American Heart Association.
“There’s an urgent need for an alternative method to monitor patients for acute heart transplant rejection,” said Sean Agbor-Enoh, M.D., Ph.D., study co-author and chief of the NHLBI’s Laboratory of Applied Precision Omics.
“We showed in our initial assessment that this ‘liquid biopsy’ is highly sensitive for detecting acute rejection, finding it weeks to months before current clinical tools. This could potentially save lives in the wake of a critical shortage of donor organs,” said Hannah Valantine, M.D., senior study author and the former lead investigator of the Laboratory of Organ Transplant Genomics in the Cardiovascular Branch at the NHLBI.
Currently a professor of cardiovascular medicine at Stanford University in Palo Alto, California, and a noted pioneer in transplantation research, Valantine said the test is also important for addressing serious health disparities. Because African Americans tend to have higher rates of heart transplant rejection and experience poorer transplant outcomes than other groups nationwide, the test could help reduce existing transplant-related health disparities that have persisted for decades.
The test, called the donor-derived cell-free DNA test, tracks DNA markers from the organ donor that appear in the blood of the transplant recipient. Because injured or dying cells from the donor organ release lots of donor DNA fragments into the bloodstream compared to normal cells, higher amounts of donor DNA indicate a higher risk for transplant rejection in the recipient. The biomarkers can be detected using specialized lab equipment.
Current tests used to detect heart transplant rejection rely on frequent and painful biopsies of heart tissue. Those biopsies run the risk of damaging the heart and are limited by their invasiveness (ability to obtain tissue samples) and reliability for detecting acute rejection.
Acute, or rapid, rejection tends to happen in the first three to six months after transplantation as opposed to chronic rejection, which occurs after many years.
In the current study, the research team collected blood samples from 171 people who had recently undergone heart transplantation at one of five regional transplant centers within or near the Washington, D.C.-metropolitan area. The sampling included nearly 2,000 cell-free DNA measurements. The population included a high percentage (about 44%) of African Americans. The researchers monitored the patients for signs of acute rejection for nearly 18 months using both traditional endomyocardial (heart tissue) biopsy and the new blood test.
The researchers found that the blood test performed better in their study than tissue biopsy, detecting higher amounts of rejection markers and earlier signs of rejection. It also detected more instances of other types of transplant injury that were missed by biopsy, including so-called antibody mediated rejection, one of the deadliest forms of rejection and the hardest to treat and diagnose. The new blood test may be able to detect rejection as early as 28 days after heart transplantation and at least three months before rejection is detectable using heart tissue biopsy.
Also, while current heart biopsies are only performed in hospitals, specimens for the new blood test can be collected at almost any commercial blood-collecting center. Since the blood test may lead to fewer biopsies, it could also provide significant financial savings. In addition, the noninvasive blood test may provide a potentially safer alternative to invasive biopsies during viral outbreaks like the current COVID-19 pandemic.
However, the new test still has limitations. “This test will not completely eliminate the need for invasive procedures, but it can eliminate about 80% of the biopsies currently performed after heart transplant,” said study co-author Palak Shah, M.D., a heart disease specialist at Inova Heart and Vascular Institute, Falls Church, Virginia.
Researchers need to conduct additional clinical studies, including a randomized controlled trial, to confirm these findings, but they are using the blood test to further study the mechanisms underlying acute rejection and explore why African Americans tend to have higher rates of transplant rejection, with the goal of eliminating this health disparity. If future testing in a clinical trial validates the safety and efficacy of the new test, it could become a routine tool used to monitor heart transplant patients for early stages of rejection, the researchers said. The exact timeline for its clinical availability is unclear, they noted.
This study was funded by the Division of Intramural Research of the NHLBI (HHSN268201300001C), part of NIH. The study also included funding from the American Heart Association/Enduring Hearts Foundation Scientist Development Grant (17SDG33660431).
Study: Cell-Free DNA to Detect Heart Allograft Acute Rejection. Circulation. DOI: 10.1161/CIRCULATIONAHA.120.049098
About the National Heart, Lung, and Blood Institute (NHLBI): NHLBI is the global leader in conducting and supporting research in heart, lung, and blood diseases and sleep disorders that advances scientific knowledge, improves public health, and saves lives. For more information, visit www.nhlbi.nih.gov.
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.